Soil Testing After a Drought
While we have not experienced a drought in 2020, there are areas of our trade area that have been abnormally dry. This article from 2012 does a great job explaining how dry weather conditions can impact soil test results.
There has been a substantial amount of information and speculation published recently regarding how the drought of 2012 may affect soil test results of samples collected during the dry period. This article is an attempt to summarize these facts.
Soil pH: Water pH readings may be 0.1 to 0.6 pH units lower than expected. This is due to a slight increase in soluble salts in the soil solution that haven’t leached into the soil profile. This condition, though, does not alter the buffer pH result so the amount of lime recommended for most samples will not be affected. An exception to this would be sandy soils where the water pH determines the lime recommendation. However, sandy soils are leached more easily so the amount of soluble salts in solution may be much lower than a heavier soil.
Potassium: Soil test levels for potassium may be lower than normal. When soils remain extremely dry for extended periods of time, the moisture that normally keeps the clay latticework open for potassium exchange retracts, capturing the available potassium from solution. This will show up as a reduction in the soil test level. Also, potassium is easily leached from crop residue following harvest. With little rainfall, this potassium reserve could remain in the tissue. One caveat of this, though, is with inadequate moisture to produce normal yields, less potassium may be removed from the soil reserve.
Phosphorus: Soil test levels for phosphorus may be slightly lower than normal. The affect of the dry soil on phosphorus levels isn’t as dramatic as potassium, but less moisture in the soil may lower the soil test readings. The same situation of reduced crop yields, though, may result in less phosphorus being removed from the soil.
Soil sampling technique: It is extremely difficult to sample dry soils. Many times the top one or two inches of the core are compressed enough that some of this material may spill out of the probe. In minimum tillage situations, this could have a dramatic affect on the soil test readings. As of the publication date of this newsletter, hurricane Isaac has deposited a substantial amount of rainfall on much of the Midwest. Soils in the lower half of Illinois, Indiana, and Ohio may have enough time to equilibrate moisture levels prior to fall sampling so that some of the drought effects will be negligible. Reduced yields, though, will still be a remnant of decreased nutrients being removed from the soil. This year is one where soil sampling should occur in order to assess the affects of this unusual growing season. Soil sampling technique: It is extremely difficult to sample dry soils. Many times the top one or two inches of the core are compressed enough that some of this material may spill out of the probe. In minimum tillage situations, this could have a dramatic affect on the soil test readings. As of the publication date of this newsletter, hurricane Isaac has deposited a substantial amount of rainfall on much of the Midwest. Soils in the lower half of Illinois, Indiana, and Ohio may have enough time to equilibrate moisture levels prior to fall sampling so that some of the drought effects will be negligible. Reduced yields, though, will still be a remnant of decreased nutrients being removed from the soil. This year is one where soil sampling should occur in order to assess the affects of this unusual growing season.
Not all Corn Nematodes are the Same
While soybean cyst nematodes have been getting most of the fanfare the past few years, corn nematodes are making their debut. While all can be damaging, some are a bit nastier than others. Their is a wide range of acceptable thresholds to treatment among the various species. The chart below gives a range of damage thresholds from several universities. While the numbers vary between sources, the pattern of the more damaging nematodes is constant. Needle and sting nematodes have the greatest potential for damage if detected.

We can analyze soil for all of these species in our N3 soil test package that provides a count for each species of nematodes with damage interpretations. For more information on sampling or sample submission contact your ALGL agronomy representative.
Sources:
https://extension.entm.purdue.edu/pestcrop/2009/issue18/PandC18.pdf
https://crops.extension.iastate.edu/cropnews/2009/04/quick-facts-about-corn-nematodes
https://extension.uga.edu/publications/detail.html?number=C834
https://grains.caes.uga.edu/content/dam/caes-subsite/grains/docs/corn/2019-Corn-Production-Guide.pdf
https://nematode.unl.edu/extpubs/nemakan.htm
https://extension.soils.wisc.edu/wcmc/species-thresholds-and-management-of-corn-nematodes/
https://nematode.unl.edu/belonolaimusspecies.html
Low K, B and S in Tissue Samples
Tissue testing is in full swing this summer and at a rapid pace. More growers and agronomists are evaluating tissue tests to learn more about the effectiveness of their fertility programs. There are some common areas of concern arising this year. As the ALGL agronomy staff review and approve tissue test data before delivery to the customer, a trend for low sulfur, potassium, or boron is developing. The calls from growers and agronomists about these nutrients confirm that they are seeing this trend as well.
Tissue tests are very good at validating whether the crop can access the nutrients in the soil. When a tissue test comes back low for a nutrient the first question becomes, is the nutrient in the soil and the plant cannot access it, or is the nutrient deficient in the soil? Therefore, a soil sample, taken in conjunction with a plant tissue sample, is very useful.
One likely reason explaining low tissue test results for these three nutrients is a deficiency in the soil. Our annual average soil test values for these nutrients have been decreasing over the past 20 years. Less nutrient in the soil increases the probability that plants may not be able to access adequate quantities. Potassium soil test levels have been decreasing at an average rate of 0.5 ppm/year over the last 23 years. Sulfur soil test levels (measuring plant available sulfate) has declined at an average rate of 0.5 ppm/year and boron has declined on an average of 0.02 ppm/year over this same 23-year time span. This may not seem like much, but the soil test values of sulfur and boron are approximately half of what they were 20 years ago. These declines are being attributed to crop removal and/or leaching, out pacing nutrient application.


The second challenge leading to limited plant access to nutrients is weather related. Dry soil conditions can reduce the movement and uptake of all three of these nutrients in the soil, and we have seen those conditions in portions of our trade area recently. Reduced root exploration of the soil due to soil compaction is also a contributing factor in many fields. Soil compaction from traffic and tillage of wet soil the past few years have led to compaction layers restricting root growth. Heavy rains can leach both sulfur and boron, and sometimes potassium in specific situations, below the resulting shallow root zone.
Soil testing is valuable tool in ensuring you have the need foundational soil fertility for a productive crop, but tissue testing identifies if the crop can access and utilize this fertility.
Shipping Made Easy
At ALGL, we strive to be a company built on integrity and being easy to work with. The same goes for our shipping program. Rather than focusing on gimmicks and promotional shipping prices we provide cost effective, streamlined, fair, and easy shipping options. Your time is better spent servicing your customers than on the logistics of shipping samples.
We offer United Parcel Service (UPS) Return Shipping (RS) labels for your shipping convenience. This allows you to take advantage of our significant shipping volume discounts. By following the steps below, you can help ensure that you are getting the best prices and service from our shipping program.
1. Your Account – You must have an up-to-date, active ALGL account to use our UPS shipping program. Be sure to contact us if any updates need to be made such as contact names, street address, e-mail address, phone number etc.
2. Order Supplies– We offer 4 convenient boxes for sample shipment. Order the boxes and associated labels online or by calling the lab. You purchase the boxes and we ship them with the labels to you.
3. Pack Your Samples – Place the samples in the box so that they will not spill in shipment, tape the box shut, and affix the UPS RS label on the box. The box can be part of your usual UPS pickup, dropped off at a UPS pickup location, or you can call UPS to schedule a pickup. There may be a cost associated with a UPS on site pickup, please inquire with your local UPS representative.
4. Invoice – You are not charged for the UPS RS labels until they are scanned by UPS upon pickup. The cost of the shipment is calculated using our discount, and that amount is transferred to your invoice. The cost of the sample analysis and shipping come to you on one invoice.
UPS RS labels can be printed at any time, and there are no charges associated with the labels until they are used. This means they can be printed in advance and ready to use when you are already to ship. One challenge in printing UPS RS labels too early is that the tracking capabilities for a given label decline 12 – 16 months after creation.
The date of creation can be tracked directly on the label. If you are nearing the expiration date printed on the UPS RS labels, you can request new labels by calling/emailing the lab, calling/emailing your ALGL agronomy sales representative, or ordering via our on-line store at www.algreatlakes.com.Rapid Growth Stage in Corn
Early corn planting dates often occur when soils are cool, night time temperatures are low and heat unit accumulation comes at a very slow pace. The first 3 to 6 weeks of a corn plant’s life can be a slow struggle with seemingly little progress but around V-4 permanent roots are becoming established and with increasing temperatures the rapid vegetative growth stage of the crop is quickly approaching.
Corn growth and physiology research performed by Purdue shows some impressive statistics about the crop’s growth potential from V-4 stage through tassel or 21 days after planting to 71 days after planting.
- Root length increased from 54 miles per acre to 32,000 miles per acre
- Above ground dry matter weight of the stover increased from 29 pounds per acre to over 9,000 pounds per acre.
- 73% of the seasonal nitrogen uptake enters the plant along with 74% of the phosphorous and 85% of the potassium.

While most of the dry matter weight added during this 50 day period is comprised of carbon, hydrogen and oxygen supplied by the air and water plant growth cannot continue without adequate supplies of essential plant nutrients. It is important to maintain proper agronomic nutrient levels and monitor these levels with the use of a good soil testing program so the soil will be able to supply the needs of the crop through this rapid nutrient uptake phase.
A good scouting program prior to tassel may reveal visual symptoms of nutrient deficiencies if soils were unable to meet the high nutrient demands during this short window of time and data provided through plant tissue testing will help reveal critical nutrients that may be in short supply.

Data from University of Illinois
Information provided by the University of Illinois suggests that the crop must successfully build a “photosynthetic factory” comprised of approximately 5 tons of dry matter per acre to be well positioned for maximum production that will follow in the reproductive stages of growth.
Know Your Irrigation Water
Irrigation water analysis focuses on the impacts that irrigation water may have on the soil. The repeated application of irrigation water can change the chemical and physical properties of the soil over time. Therefore, the interpretation of data from irrigation water analysis is driven by the prediction of the effects of the irrigation water source on the soil.

The chemical properties of irrigation water drawn from a well is greatly influenced by the bedrock type surrounding the aquifer, as well as the composition of the aquifer itself. For example, an irrigation well drawing water from a limestone bedrock high in calcium will most likely have a high pH, high calcium content, will be high in carbonates, and contain elevated dissolved solids. However, not all limestone is the same. Also, there can be different layers within the bedrock leading to differences in irrigation water quality with the depth of the well.
Good long-term management of irrigated land needs to take irrigation water quality into consideration. The repeated use of irrigation water with a high concentration of a specific element or compound can lead to accumulations and potentially excess levels of that element or compound in the soil. Depending on the specific material, this could lead to reduced plant health and/or yield over time. For example, the repeated application of irrigation water with high calcium carbonates without acid injection treatment can lead to plugging of irrigation equipment and the continual increase in soil pH over time. The increasing soil pH, if left unmanaged, could become high enough to limit the availability of plant nutrients, as well as lead to other negative management challenges. This is of greater concern if the same bedrock influenced the formation of the soil. In this example, the soil would also be inherently higher in carbonates and have a higher pH before additional calcium carbonates are added to the soil through the irrigation water.
For sound management of irrigated land, irrigation water analysis is a crucial tool to identify possible issues associated with the irrigation water source, and to define proper techniques to mitigate those issues. However, proper management also requires routine soil testing to monitor the impact irrigation water has on the soil over time to ensure the overall irrigation water management is working.
Sampling for SCN
Interest has been steadily growing in soil sampling for Soybean Cyst Nematode (SCN), and with good reason. SCN continues to be the leading yield loss pathogen in U.S. soybean production. The impacts of SCN continue to grow as the pest continues to spread throughout the soybean production acres of the U.S. The map below shows how SCN has spread from a small isolated area along the Mississippi River in 1957 to the last survey in 2014. It is particularly concerning how quickly the area affected has expanded since 2001.
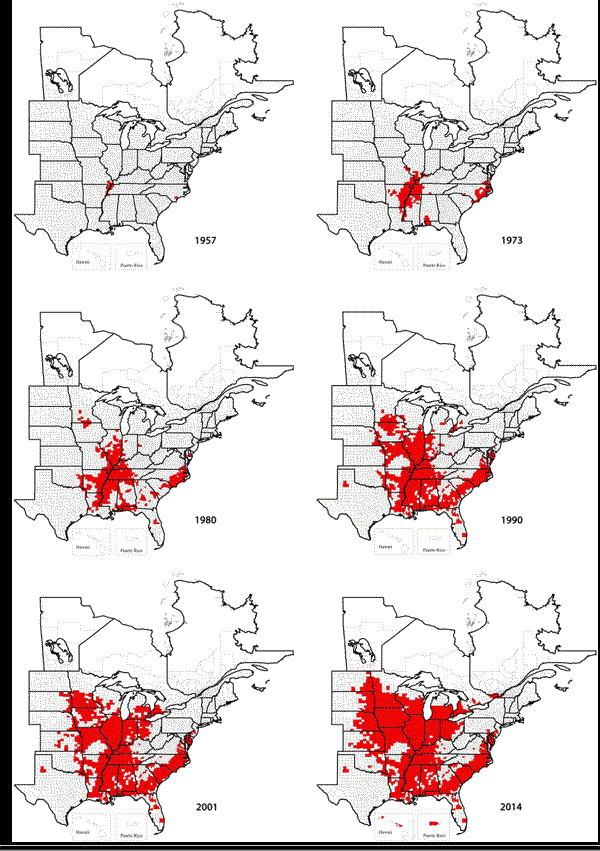
https://www.soybeanresearchinfo.com/diseases/scnpics/SCN_dist57_14_lg.gif
The spread of SCN through the Great Lakes region, increased focus on high yield soybeans, the potential link of Sudden Death Syndrome to plants experiencing SCN feeding, and new products on the market showing some level of SCN control has increased the interest in sampling for SCN.
Sampling for SCN can take two forms: a diagnostic approach to identify a crop issue, or a proactive management approach looking at whole field SCN levels to determine future planned management activities. Each of the approaches have different sampling procedures and interpretations, but utilize the same laboratory procedures. A N-CYST test from A&L Great Lakes Laboratories provides a count of both SCN eggs and adult SCN cysts which are used to identify treatment and management thresholds.
A diagnostic approach is used when a yellow and stunted area of a soybean field is suspected to have elevated SCN populations leading to the visual symptoms. In this case, soil sampling for SCN will be targeted to verify the presence and amount of SCN in the affected area. While visual inspection of the roots can note the presence of SCN, it does not quantify the population. SCN may be present, but at populations below the threshold at which injury should occur. To properly sample for SCN, 8 or more soil sample cores should be taken 6 to 8 inches deep in the affected area. If the field has a history of elevated SCN levels it may be advisable to take a sample from a portion of the field not showing visual symptoms to collect comparative data. Place the soil cores in a clean plastic bucket. Once all of the cores are collected, thoroughly mix the sample and place two cups of soil into a sealed and labeled soil sample bag or plastic bag. The samples should be sealed to avoid moisture loss and protected from extreme temperatures; do not freeze or refrigerate, or leave in the dash of the truck on a summer day. A cooler can be very helpful for sample storage during collection. If the samples are handled in such a way that lead to cyst death, the adult counts will be negatively impacted. Ship or deliver to the lab a quickly as possible.
As a tool for proactive management of SCN, whole field samples can be collected to identify average SCN populations across a field or region of a field. This method is helpful in identifying fields that need additional management to address SCN, but populations can be underestimated when sampling a large area, because small areas of very high SCN populations can be diluted with unaffected areas. Whole field sampling for SCN mirrors traditional whole field composite soil fertility samples. Take samples late in the growing season after flower through harvest. Collect a minimum of 10 to 20 soil cores to a depth of 6 to 8 inches, while walking in a zig-zag pattern across the field, and place the soil cores in a clean plastic bucket. Once all of the cores are collected, thoroughly mix the sample and place two cups of soil into a sealed and labeled plastic bag. Again protect the samples from drying out and from extreme temperatures while shipping the samples to the laboratory as quickly as possible.
For any additional questions regarding SCN sampling, feel free to contact your A&L Great Lakes Laboratories agronomist or call the laboratory directly at 260-483-4759.Making Sense of Soil Nitrate and Ammonium Values
We have been fielding a wide variety of questions around soil nitrate and ammonium soil test levels. Many soil nitrogen levels from fall manure applications are indicating the need for supplemental nitrogen. Wet weather, and brief periods of warm weather, have led to nitrogen loss. When manure was applied later in the spring, the results are looking much more positive, often above the 25 ppm nitrate level that is universally considered adequate to produce a corn crop. There are some soil tests near or just below the 25 ppm nitrate threshold and may need to be reevaluated later in the season for a possible late season nitrogen application. This re-evaluation later in the season should allow time to evaluate nitrogen loss due to weather the remainder of the season and provides the opportunity to determine a realistic yield expectation. More information on interpretation of Presidedress Soil Nitrate Testing (PSNT) for Corn can be found on our website.
The traditional PSNT interpretations can be challenging to relate to. Providing that the samples were collected to a depth of 12” there is a simple “rule of thumb” to help make sense of a soil nitrate and ammonium value. Add the ppm of the nitrate and ammonium together and multiply by four. This is a relative nitrogen application rate available in the soil at the time of sampling. For example, sample 1 below would be 20 ppm nitrate + 10 ppm ammonium = 30 ppm x 4 = 120 pounds nitrogen. More on this concept can be found at https://www.agry.purdue.edu/ext/corn/news/timeless/AssessAvailableN.html.

While the traditional PSNT interpretations assume a continuous release of nitrogen to the soil from the mineralization of manure organic materials during the growing season, this calculation helps relate to a nitrogen level in the soil today. The difference between the estimated pounds of nitrogen, and a total nitrogen program for the season, is about the same as the PSNT interpretations would recommend.
Spring 2020: Heating Up
Some areas of the corn belt experienced good field conditions in late March and early April that allowed for fertilizer applications, burn down spraying, and other field preparation. In many areas, conditions were very tempting for planting but the temperatures and the calendar were a concern. In the southern part of the region, some corn acres were planted during the first week of April with additional acres planted about two weeks later. Just as spring looked to be well on track, along came the cold nights in early May with widespread freeze damage for many growers.
If we take a closer look at the development of a corn crop, assuming a planting date of April 20 and moving to the present date of May 25, average growing degree unit accumulation for central Indiana would be about 11.8 units/day. Over that time period, we would be approaching a total of 413 units. In 2020, according to the University of Illinois heat unit calculator, Hendricks County Indiana near Indianapolis has only received 4.2 heat units/day during that period and we are now at a deficit of 265 heat units compared to the 30 year trend line.
The following diagram tracks heat unit accumulation in 2020 on the black line compared to the 30 year average plotted in purple. The data would indicate corn planted April 20 at this location emerged in about 17 days on May 6 and at the present time crop development is tracking about 15 days later than normal. The delayed emergence might have caused some minor stand loss and the reduced leaf area, and transpiration rate. In addition, reduced root mass due to the delayed development may limit nutrient uptake rates until temperatures return to a warmer seasonal pattern. Mineralization of soil organic matter will occur at a slower rate and subsequently availability of mineralized nitrogen, sulfur, and boron may be observed.

Post emerge herbicide applications may be pushed a few days later than normal and weed development will also be delayed by the cooler temperatures, and extra care will be needed to properly time these activities for best weed control. Rapid growth phase of the crop and maximum nutrient uptake may be slightly later than normal, depending on temperatures moving forward.
The next diagram shows how the crop might progress with a return to normal 30 year trend GDU accumulation. Warmer, sunny days ahead may allow the crop to return to a more average rate of development.

University of IL Growing Degree Calculator
Collecting Plant Tissue Samples
We put a great deal of effort and resources into ensuring quality with our analyses here at A&L Great Lakes Labs. We want the data that you receive from us to be of the highest quality so that it is of the most benefit to you and your operation. However, quality analysis is only one piece of the puzzle. Good quality data begins with a good quality sample, and how the sample is collected and handled after collection goes a long way to ensuring its usefulness.
Plant tissue testing can be a very valuable tool to use in your fertility program. However, there are a number of guidelines that should be followed to ensure that this information is useful to you.
- Sample the correct part of the plant. The interpretations of plant tissue analysis have been developed based on a particular part of the plant, and that part can vary based on the crop and growth stage of the crop. For more information, please refer to our Plant Analysis Sampling Guide, available from our website.
- Collect enough sample for analysis. The amount of sample to collect can also be found in the Plant Analysis Sampling Guide. The amount of material listed is generally a guideline to help ensure that the sample is representative, but is not a minimum requirement.
- If the samples are extremely dirty, shake off any excess dirt or gently wipe the samples off. Washing of samples is generally discouraged, as this can affect the potassium (K) content of the material. If you do choose to wash the samples, do so soon after sampling and before shipping to the lab to reduce these losses as much as possible.
- Place the samples into PAPER bags, never plastic! Paper bags allow the samples to breathe and preserve the integrity of the sample.
- Include a completed Corn and Soybean Plant Submittal Form or Plant Tissue Submittal Form (for all other crops) with your samples. Complete the form as thoroughly as possible to ensure that your report is accurate. Be sure to indicate the plant type and growth stage on the submittal form.
- Pack the samples loosely into a box, and ship them to the lab as soon as possible. It is generally best to ship the samples so that they arrive at the lab within 2 days (samples shipped via UPS Ground generally arrive within two days when shipped from anywhere in the Great Lakes region). It is best to ship samples Monday-Wednesday, to reduce the possibility of samples being in transit over the weekend.
If you have any questions about plant tissue analysis, please contact your A&L Great Lakes regional agronomist or call the lab at 260-483-4759 and we will be happy to assist you!



